Larviciding is seen as an important tool to tackle vector-borne diseases where LLINs and IRS are not sufficient to eliminate mosquito populations. Larvicides are able to target outdoor resting and biting mosquitoes which are less affected by LLINs and IRS and are often used in tandem with them as part of an insecticide resistance management strategy.
Larviciding targets the immature stages (larvae and pupae) of the mosquito vector and aims to lessen the number that reaches adulthood, thus reducing the transmission of vector-borne diseases. Larval insecticide bioassays expose laboratory-reared mosquito larvae of known age or instar for 24-48 hours or longer in water treated with larvicide to evaluate its biological activity. Various concentrations within the larvicides activity range are tested, and mortality is recorded.
The aims of performing a larval bioassay include, but are not limited to:
- To establish dose-response line(s) against susceptible vector species;
- To determine the lethal concentration (LC) of the larvicide for 50% and 90% mortality (LC50 and LC90)
- To determine the lethal concentration for 50% and 90% inhibition of adult emergence (IE50 and (IE90)
- To assess cross-resistance with commonly used insecticides
- To establish a diagnostic concentration for monitoring susceptibility to the mosquito larvicide in the field
What types of larvicides do we test?
- Chemical larvicides
- Insect Growth Regulators (IGRs)
- Bacterial/Fungal Larvicides
We can procure a variety of mosquito larvicides, or clients can provide us with their own.
What species of mosquitoes can be tested?
LITE houses a variety of susceptible and resistant colonies of Anopheles spp., Aedes sp., and Culex sp. that are mass-reared for testing purposes.
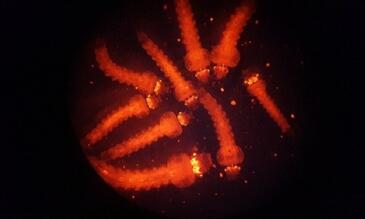
Previous larval insecticide bioassays involved rearing, back-crossing and screening transgenic mosquitoes for larvicide testing (Figure 1)
Clients may request one of LITEs susceptible/resistant colonies or provide a specific colony for us to rear, maintain and subsequently test.
Results
All data is recorded and provided to the client, and the appropriate statistical analysis conducted. Results are given in a full report to the client.
Figure 1. Previous larval insecticide bioassay performed in LITE (left) using transgenic An. Coluzzi marked by the DsRED fluorescent marker in the eye and nervous system.
WHO Guidance & Published Methods:
All larval bioassays conducted in LITE follow our Standard Operating Procedure LITSOP011 ‘The performance of Insecticide Larval Bioassays’. The SOP is founded on guidelines from the World Health Organisation (WHO) ‘Guidelines For Laboratory and Field Testing of Mosquito Larvicides’:
World Health Organization, 2005. Guidelines for laboratory and field testing of mosquito larvicides (No. WHO/CDS/WHOPES/GCDPP/2005.13). World Health Organization.
